More Information
Submitted: February 19, 2021 | Approved: March 02, 2021 | Published: March 03, 2021
How to cite this article: Noory E, Böhme T, Beschorner U, Zeller T. Bleeding complications at the access sites during catheter directed thrombolysis for acute limb ischaemia: Mini review. Arch Vas Med. 2021; 5: 001-003.
DOI: 10.29328/journal.avm.1001014
Copyright License: © 2021 Noory E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Acute limb ischemia; Peripheral artery disease; Thrombectomy; Catheter directed thrombolysis; Protection device; Bleeding complications
Bleeding complications at the access sites during catheter directed thrombolysis for acute limb ischaemia: Mini review
Elias Noory*, Tanja Böhme, Ulrich Beschorner and Thomas Zeller
Clinic for Cardiology and Angiology II, University Heart Centre, Freiburg- Bad Krozingen, Germany
*Address for Correspondence: Dr. Elias Noory, Clinic for Cardiology and Angiology II, University Heart Centre, Freiburg- Bad Krozingen, Südring 15, D-79189 Bad Krozingen, Germany, Tel: +49 7633 402 2431; Email: [email protected]; [email protected]
Acute and subacute ischemia of the lower limbs represents a major emergency with a high in-hospital mortality, complication, and leg amputation rates.
Treatment options for acute limb ischemia include systemic anticoagulation, followed by various catheter based options including infusion of fibrinolytic agents (pharmacological thrombolysis), pharmacomechanical thrombolysis, catheter-mediated thrombus aspiration, mechanical thrombectomy, and any combination of the above or open surgical intervention (thromboembolectomy or surgical bypass).
Minor and major bleeding complication during catheter directed thrombolysis (CDT) especially at access site are frequent. Bleeding complications require often an interruption or termination of CDT affecting clinical outcome of the patients. Recently we examined a new access site bleeding protection device during CDT.
Catheter directed thrombolysis or surgery
Acute lower limb ischemia is caused by a reduction of arterial perfusion to the limb due to either embolic occlusion or local thrombosis. To date no clear therapy recommendations exist.
Three randomized, multicentre trials were published comparing thrombolysis with surgery: the Rochester study [1], the Surgery versus Thrombolysis for Ischemic Lower Extremity (STILE) trial, [2] and the Thrombolysis Or Peripheral Arterial Surgery (TOPAS) trial [3]. The consensus that emerged from these studies and others was that CDT should be considered as first-line treatment for acute limb ischemia under the following conditions: (1) symptoms of limb ischemia present for < 14 days, (2) no absolute contraindications to thrombolysis, and (3) the predicted time to re-establish antegrade flow is short enough to preserve limb viability. Barring contraindications, CDT is indicated urgently for patients with marginal limb threat (category IIa according to the TASC criteria). Patients with immediate limb threat (category IIb) often require emergent operation as thrombolysis alone will not re-establish perfusion quickly enough to salvage the limb. Nonetheless, surgery incurs the risk of systemic effects of rapid reperfusion with compromise of cardiac, pulmonary, and renal functions that may result in prolonged hospitalization or even death [4,5]. If the cardiovascular risks of general anaesthesia due to comorbid disease and status of the affected limb outweigh the risk of a delay in reperfusion, then thrombolysis may be the best option. Last resort specialised interventional procedures and primary amputation is the only option permitting survival for patients with irreversible ischemia (category III). High perioperative mortality is associated with this degree of acute ischemia.
CDT and access site bleeding complications
Catheter-directed thrombolysis (CDT) is an attractive non-surgical option for many patients with acute critical ischemia, which involves the insertion of an infusion catheter (via the femoral artery) in the catheterization laboratory to reach the thrombus to allow the delivery of the pharmacological thrombolytic agent. Either an antegrade (in the direction of the blood flow) femoral artery puncture, or a retrograde route from the contralateral femoral artery can be used. Both bolus and continuous thrombolytic therapy may be performed. A variety of agents can be used in either low or high dose regimens. Urokinase and (recombinant) tissue plasminogen activator (rtPA) are the most effective agents, but neither of these have been shown to be superior in terms of efficacy and bleeding complications [6]. Following catheter insertion, the patient undergoing CDT is usually transferred to an intermediate or intensive care unit, with the delivery catheter in place, and the infusion running for up to 48 hours. Ebben undertook a review of studies comprising a total number of 10,643 cases of which 9877 received CDT for lower extremity arterial occlusion, with a mean treatment duration of 21.4 h and a freedom from amputation rate of 91%. Pooled results showed a thrombolysis duration with high dose protocols of 21.9 h, and 32.7 h with low dose protocols [7].
The major complications reported with CDT include major bleeding, and early termination of the CDT due to access site bleeding. Bleeding complications remain an important risk of CDT with bleeding rates in 18% (95% CI 17.8-18.3) of patients with bleeding rates of high dose protocols 16.7% (95% CI 16.3-17.1) and in low dose protocols of 13.4% (95% CI 12.8-14.0). Three factors associated with the bleeding risk are identified: the arterial puncture, the length of the CDT infusion with the sheath remaining in the femoral artery, and the thrombolytic agents leaking into the circulation, and having a systemic effect [8].
Abandonment or interruption rate of the CDT procedure can occur if complications are observed. Rates of interrupted CDT procedures due to bleeding range from 5% to 11.5% [9-11]. Definitive control of the access site remains an ongoing problem [12].
Protection devices for reduction of access site bleeding complications during CDT
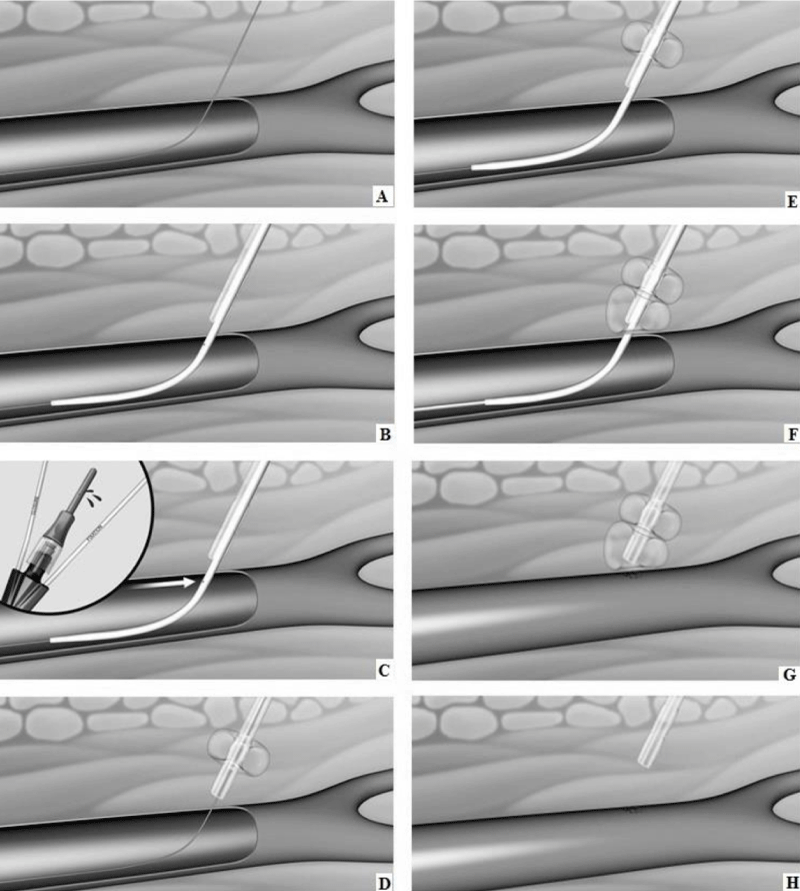
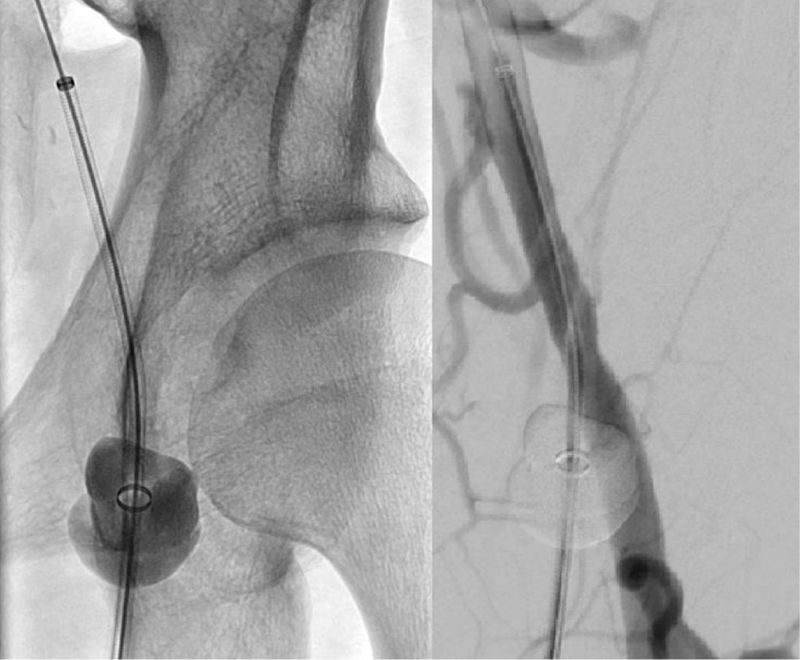
Currently, there are no devices on the market that are approved and indicated for vascular access site bleeding protection during CDT in patients diagnosed with acute critical limb ischemia. CaveoMed developed the CaveoVasc® Thrombolysis Protection System, a vascular access protection device for use in thrombolysis procedures. Its function is to facilitate sheath access, and minimize risks of access site bleeding complications during lengthy catheter-directed thrombolysis procedures. Pressure balloons inflated outside the artery maintain a tight seal of the arterial puncture site throughout thrombolysis. After removing the thrombolysis catheter the protection sheath is removed, and hemostasis at the access site is managed as per the hospital’s standard procedure with a closure device or other techniques. A schematic representation of the use of the CaveoVasc® Thrombolysis Protection System is given in figure 1. An angiogram after placement of the CaveoVasc® Thrombolysis Protection System is shown in figure 2. The device got CE Mark June 2020.
Figure 1: Schematic representation of CaveoVasc® procedure. A. Guidewire in femoral artery. B. CaveoVasc® is applied after punction of the femoral artery and placement of the guide wire, thus in the beginning of the procedure. C. Removal of Locator, blood backflow indicates correct position. D. Infaltion of the Fixation Balloon – the inflated balloon secures the position of CaveoVasc® in the tissue. E. Placement of the sheath. F. Inflation of Pressure Balloon – Start of thrombolysis therapy via catheter perfusion. G. Removal of the sheath and catheter after the end of the thrombolysis procedure. H. Removal of CaveoVasc®.
Figure 2: Angiogram with the CaveoVasc® protection system. Angiogram of the left common femoral artery with the CaveoVasc® protection system.
Essentially, the system is intended to protect the patient from peri-procedural bleeding at the arterial access site, which currently remains a major problem for patients with critical limb ischemia undergoing CDT treatment. Recently we published the results from the first in men study demonstrating that the bleeding protection device lowered the rate of major bleeding events at puncture sites [13].
Catheter-directed thrombolysis (CDT) is an attractive non-surgical option for many patients with acute limb ischaemia. Bleeding complication especially at the access site remains a major unsolved problem, reducing procedural success rates and contributing considerably to periprocedural mortality and morbidity.
Successful maintaining femoral access for long periods, up to as long as 40 hours, is still a challenge, especially in patients being treated with anticoagulation and thrombolytic drugs. Definitive control of the access site remains an ongoing problem.
The CaveoVasc® Thrombolysis Protection System can be used as add-on to current thrombolysis therapy. It has demonstrated a reduction of bleeding complications in CDT by protecting the puncture site by stabilizing the sheath with the double balloon technique and tamponing minor bleeding in the puncture channel. The application is simple and requires only a few additional intervention steps.
- Ouriel K, Shortell CK, De Weese JA, Green RM, Francis CW, et al. A comparison of thrombolytic therapy with operative vascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994; 19: 1021–1030. PubMed: https://pubmed.ncbi.nlm.nih.gov/8201703/
- Robert. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischaemia of the lower extremity. The STILE trial. Ann Surg. 1994; 220: 251–268. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1234376
- Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPASInvestigators.J Vasc Surg. 1996; 23: 64-73. PubMed: https://pubmed.ncbi.nlm.nih.gov/8558744/
- Diffin DC, Kandarpa K. Assessment of peripheral intra-arterial thrombolysis versus surgical revascularization in acute lower-limb ischemia: a review of limb-salvage and mortality statistics. J Vasc Interv Radiol. 1994; 7: 57–63. PubMed: https://pubmed.ncbi.nlm.nih.gov/8773976/
- Beyersdorf F, Matheis G, Kruger S. Avoiding reperfusion injury after limb revascularization: experimental observations and recommendations for clinical application. J Vasc Surg. 1989; 9: 757–766.
- Robertson I, Kesse DO, Berridge DC. Fibrinolytic agents for peripheral arterial occlusion. Cochrane Database Syst Rev. 2013; 12: CD001099. PubMed: https://pubmed.ncbi.nlm.nih.gov/24357258/
- Ebben HP, Jongkind V, Wisselink W, Hoksbergen A, Yeung KK. Catheter Directed Thrombolysis Protocols for Peripheral Arterial Occlusions: a Systematic Review European Journal of Vascular & Endovascular Surgery, Eur. J.Vasc. Endovasc. Surg. 2019; 57: 667-675. PubMed: https://pubmed.ncbi.nlm.nih.gov/31005512/
- Verhamme P, Heye S, Peerlinck K, Cahillane G, Tangelder M, et al. Catheter-directed thrombolysis with microplasmin for acute peripheral arterial occlusion (PAO): an exploratory study. Int Angiol. 2012; 31: 289–296. PubMed: https://pubmed.ncbi.nlm.nih.gov/22634985/
- Kühn JP, Hoene A, Miertsch M, Traeger T, Langner S, et al. Intraarterial Recombinant Tissue Plasminogen Activator Thrombolysis of Acute and Semiacute Lower Limb Arterial Occlusion: Quality Assurance, Complication Management, and 12-Month Follow-Up Reinterventions. Am J Roentgenol. 2011; 196: 1189–1193. PubMed: https://pubmed.ncbi.nlm.nih.gov/21512091/
- Grip O, Kuoppala M, Acosta S, Wanhainen A, Åkeson J, et al. Outcome and complications after intra-arterial thrombolysis for lower limb ischaemia with or without continuous heparin infusion. Br J Surg. 2014; 101: 1105–1112. PubMed: https://pubmed.ncbi.nlm.nih.gov/24965149/
- Plate G, Jansson I, Forssell C, Weber P, Oredsson S. Thrombolysis for Acute Lower Limb Ischaemia—A Prospective, Randomised, Multicentre Study Comparing Two Strategies. Eur J Vasc Endovasc Surg. 2006; 31: 651–660. PubMed: https://pubmed.ncbi.nlm.nih.gov/16427339/
- Ricco JB, Schneider F. Angio-Seal hopes for antegrade puncture require better evidence. Eur J Vasc Endovasc Surg. 2014; 48: 226–227. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24951375
- Noory E, Beschorner U, Zeller T, Böhme T. A new protection system to avoid major bleeding at puncture sites -results from the first in men study. Cardiol Cardiovasc Med. 2000; 4: 717-728.